 UDI (Unique Device Identification) coding is the new standard unique identification of medical devices, which will improve and refine the tracking and the level of security for each product, especially during the distribution phase. In 2013, the International Medical Device Regulators Forum (IMDRF) studied, created and promoted the UDI guidance document to consistently standardize it around the world. The first country to adopt this new standard was the United States on America, but it was recently adopted also within the European Union.
UDI (Unique Device Identification) coding is the new standard unique identification of medical devices, which will improve and refine the tracking and the level of security for each product, especially during the distribution phase. In 2013, the International Medical Device Regulators Forum (IMDRF) studied, created and promoted the UDI guidance document to consistently standardize it around the world. The first country to adopt this new standard was the United States on America, but it was recently adopted also within the European Union.
Medical device manufacturers have a legal obligation to ensure that each individual product is marked with a unique code that will provide the supply chain management system with key information about the product itself such as: where and when it was produced, location and so on. Each medical device must be marked with a unique code that faithfully complies with the requirements of this standard and the lack of this unique code will therefore have legal repercussions on the manufacturers themselves.
The identification standard provides for a classification into three types of medical devices:

UDI markings features
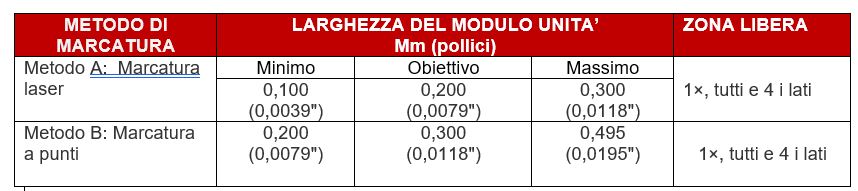
The UDI code must be applied in different ways, on packaging or, much more often, on the device itself during the production phase, through the direct marking of the so-called “GS1 code“, of two types:
The information required that must be reported in the code are the following:
These two-digit numbers are called AI (application identifiers). If numbers are to be expressed as visible characters, they must be enclosed in parentheses ().
Although UDI coding is a global standard, designed to be valid and shared with all agencies and manufacturers, you can find more details on the rules in the US and the EU at these links:
Automator lasers allow you to mark Datamatrix codes on all materials for medical devices, such as metal, alloys, plastics and polymers, including innovative materials developed specifically for this type of applications. Automator laser markers are able to guarantee the complete readability of codes even in very small dimensions, according to the standards imposed by the UDI regulations. DISCOVER OUR LASERS FOR UDI MARKING